Ever wondered what the risk is of Acute Kidney Injury (AKI) in patients with Staphylococcus Aureus Bacteremia (SAB)? I did not, and I didn’t know.
The question has been addressed in a large prospective cohort of 453 patients with SAB in seven Dutch hospitals. AKI was defined according to a modified version of the Kidney Disease Improving Global Outcomes (KDIGO) criteria for AKI which includes an increase in serum creatinine and urinary output criteria.
The cumulative incidence of AKI during the 90-day follow-up was 43%.
Sometimes a picture says more than a thousand words:
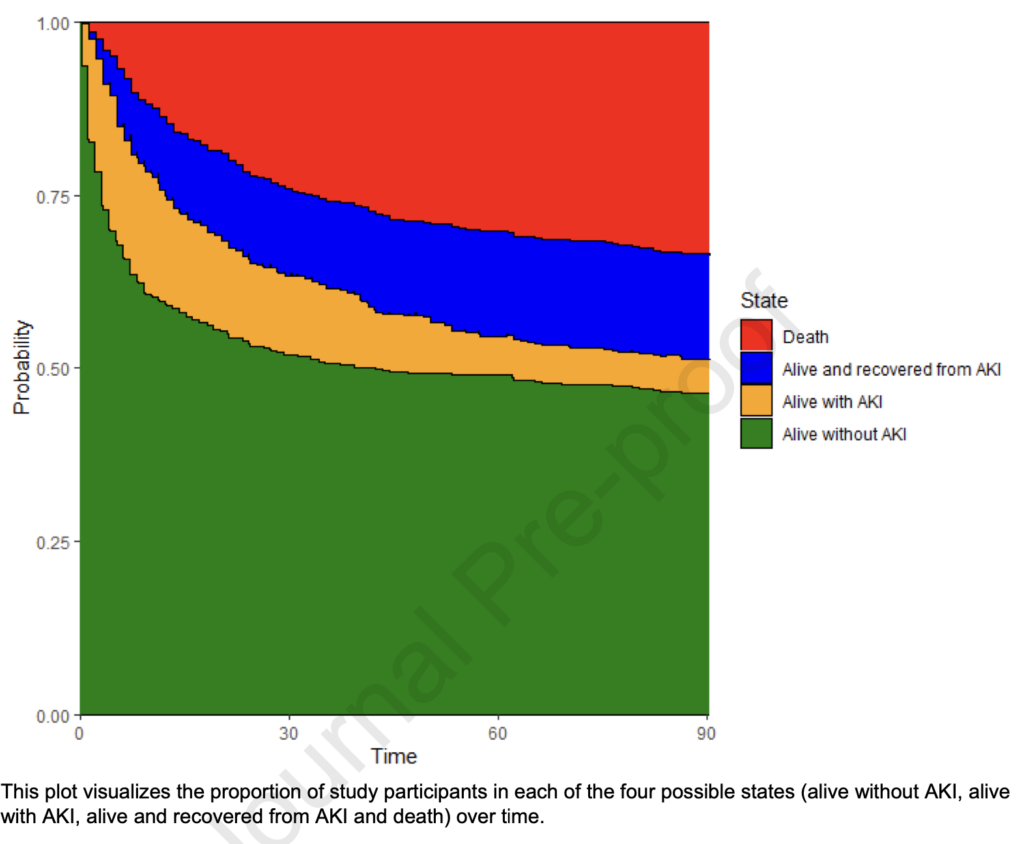
With SAB, your likelihood to be alive without (having had) AKI is less than 50% three months after infection. Age (HR 1.013, 95% CI 1.001 – 1.024), Charlson comorbidity index (HR 1.07, 95% CI 1.01 – 1.14), prior chronic kidney disease (HR 1.76, 95% CI 1.28 – 2.42), septic shock (HR 3.28, 95% CI 2.31 – 4.66), persistent bacteremia (HR 1.53, 95% CI 1.08 – 2.17) and vancomycin therapy (HR 1.80, 95% CI 1.05 – 3.09) were independently associated with AKI.
All-cause mortality at 90 days was 54% in the AKI group and 18% in the non-AKI group. After adjustment for confounders and immortal time bias, AKI was associated with an increased risk of 90-day mortality (HR 4.26, 95% CI 2.91 – 6.23).
Could AKI be caused by antibiotics? Vancomycin was used in 18% of all patients and associated with AKI, as expected. Flucloxacillin was used in 88% and cefazolin in 15% of all patients, and both were not associated with AKI, which contrasts findings from other studies, especially for the use of the anti-staphylococcal penicillins compared to cephalosporins. Yet most of these studies were retrospective, with AKI being treated as a time-fixed variable in the analysis. Moreover, those studies, as this study, may suffer from residual confounding.
Whether the predominant use of flucloxacillin for the treatment of SAB in Dutch hospitals has contributed to the – for me – unexpectedly high incidence of AKI will hopefully be answered by the randomsed comparison of flucloxacillin to cefazolin in the SNAP study. This adaptive platform trial has recruited 2,923 patients so far and is expanding to Europe with Ecraid’s help.