One week ago, the first patient with an mpox (monkeypox) infection was enrolled in the EPOXI trial evaluating the effectiveness of tecovirimat in EU countries. Then yesterday, the WHO declared the current mpox outbreak in Africa a global public health emergency for the second time in two years. And today, the first – rather disappointing – results of a randomised placebo-controlled trial of tecovirimat for mpox infection in the Democratic Republic of Congo (DRC) were announced.
Never a dull moment in trial-land.
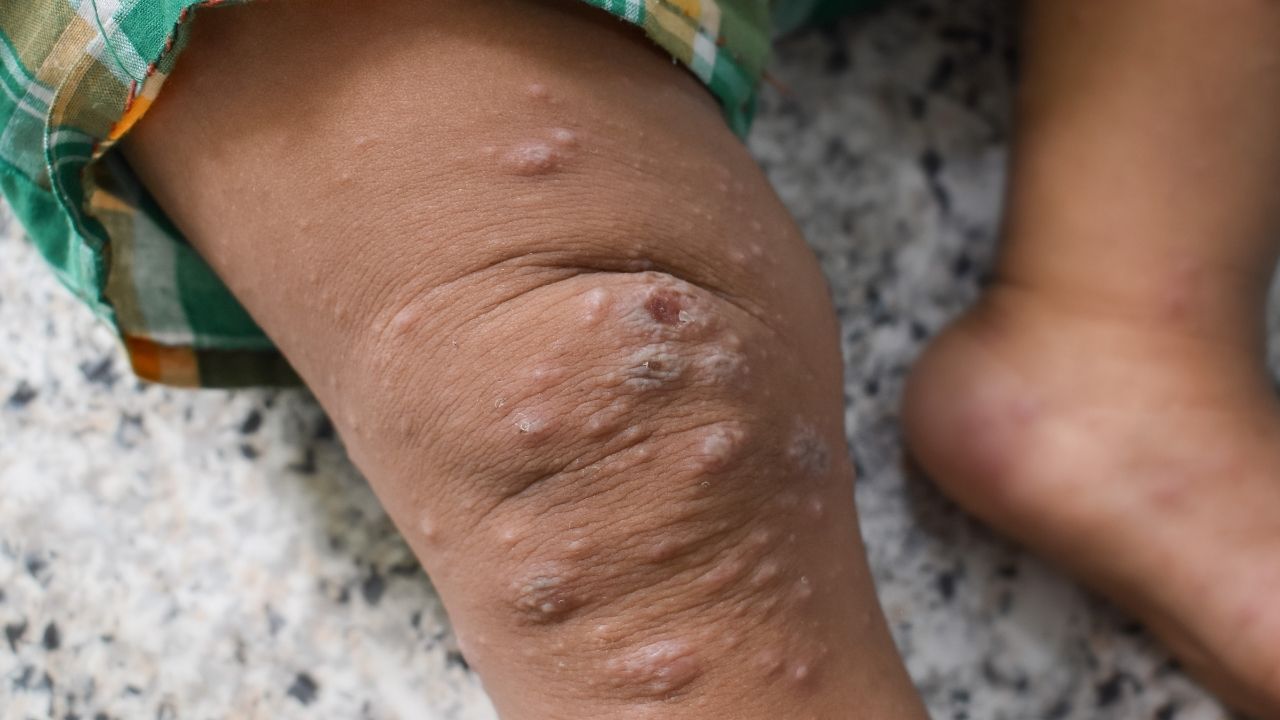
Mpox has occurred in West, Central and East Africa for decades, with the first human case identified in 1970. Two types of the virus that causes mpox have been identified. Clade I is endemic in Central Africa and can cause severe illness. Clade II is endemic in West Africa and tends to result in milder illness. A clade II subtype caused a global mpox outbreak in 2022, in Europe mostly among men who have sex with men.
The PALM007 study was initiated in October 2022 and enrolled 597 people with laboratory-confirmed mpox (clade I) at two sites in the DRC. Study participants were randomly assigned to receive tecovirimat or placebo and were admitted to a hospital for at least 14 days, where they were monitored closely for safety and resolution of mpox lesions. Tecovirimat did not reduce the duration of mpox lesions among children and adults with clade I mpox infection. More results will follow.
The trial was sponsored by the NIH and co-led by the DRC’s Institut National de Recherche Biomédicale (INRB). Without having seen any details, completing such an intensive study with almost 600 patients in less than two years is a remarkable achievement. Apparently, the Pamoja Tulinde Maisha, or PALM, clinical research partnership resulted from an earlier public health emergency response – the 2018 Ebola outbreak in DRC. This demonstrates the value of maintaining research infrastructure and collaborations to address future outbreaks.
What to do with ongoing studies evaluating tecovirimat out of Africa? “Given the differences in populations affected by the two mpox clades, the types of clinical disease that are appearing and the ongoing spread of both clades, it’s very important that we continue other related studies, so that we can develop treatments that benefit all people with mpox”, said NIAID Director Jeanne Marrazzo.
Such other studies evaluating tecovirimat in patients with mpox clade II infections are STOMP conducted in 76 sites in north and south America, Asia and Africa (NCT05534984), UNITY in Argentina, Brazil and Switzerland, and EPOXI whose first sites opened last week in Belgium and Germany, with more to follow soon in France, Germany, Spain, Portugal, Norway, and Italy. UNITY and EPOXI use similar endpoints and share DSMBs to enhance their effectiveness in reaching reliable conclusions.