Antibiotic resistance is the Darwinian consequence of antibiotic use, and poor infection control in healthcare settings its Semmelweisian accelerator. The most recent mega-meta-analysis revealed that the burden due to AMR had not really increased over the last 20 years but is expected to rise in the coming years. One of the solutions, next to vaccines and better infection control in healthcare settings, is new antibiotics. Especially for Carbapenem-resistant Gram-negatives (CR-Gn).
Yet determination of safety and efficacy of new antibiotics requires randomised clinical trials (RCTs), and the true added value of such new drugs is best determined in patients with infections caused by CR-Gn. But where to find these bacteria? This question is often a challenge.
The just-published REVISIT study aimed “to evaluate the efficacy and safety of aztreonam–avibactam (with or without metronidazole) compared with meropenem (with or without colistin) in the treatment of serious infections due to, or suspected to be caused by, Gram-negative bacteria, including metallo-β-lactamase-producing pathogens”. Between 2018 and 2023, 422 patients were enrolled at 80 sites in 20 countries; 271 patients had at least one Gram-negative cause of infection identified, 92% Enterobacterales, mostly E. coli. In all, carbapenamase enzymes were detected in 19 isolates (metrics: four per year, one per four countries, one per two sites).
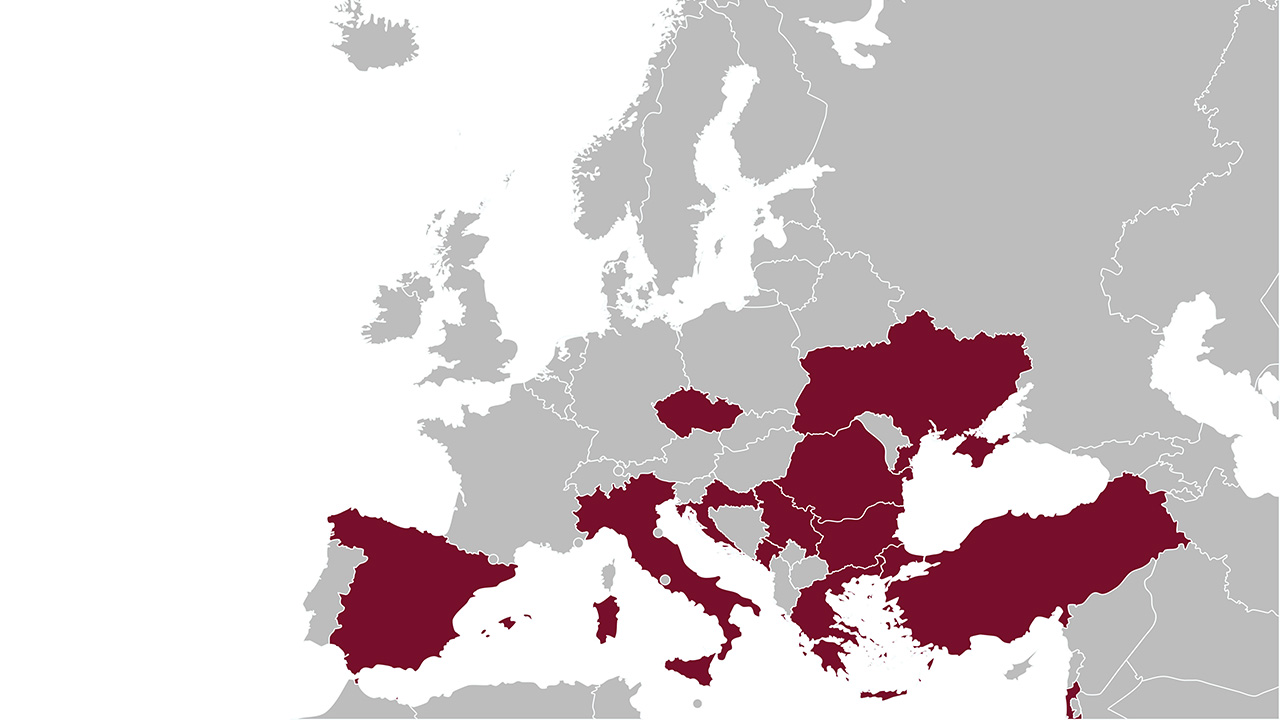
This global phase 3 study was part of the IHI-funded COMBACTE-CARE project. The COMBACTE projects aimed to establish a European clinical trial network that enhances efficiency of clinical evaluation of new antibiotics. COMBACTE-CARE included three studies on multi-drug resistant Gram-negative infections, and building the network was like “building the plane while flying”. That network, CLIN-Net, is now part of Ecraid.
The three COMBACTE CARE studies were:
EURECA: An observational study of 732 patients with CRE infection, 235 controls with CSE infections, and 705 noninfected controls in 50 hospitals in 10 countries;
REJUVENATE: A phase 2 RCT for aztreonam/avibactam with 34 patients with complicated intra-abdominal infections in 11 hospitals in three countries;
REVISIT: The COMBACTE-CARE consortium selected 38 study sites in 10 countries with high prevalence of CRE in Europe and Ukraine.
Important lessons were learned. Although journals are loaded with manuscripts on CR-Gn, it’s challenging to find such an infection and have that patient – in a short time window – enrolled in an RCT. Epidemiology helps and will tell you to go for Greece instead of Scandinavia. But which hospitals to select in Greece? Or Bulgaria? Local epidemiology is one thing, local capability to identify eligible patients, obtain informed consent, and have the drugs prepared and administered is an entirely different thing.
Therefore, my vision is that we need a warm-base network with perpetual studies that can be used for challenging but extremely important clinical studies, such as those with new antibiotics for CR-Gn. Detailed information about national and local epidemiology, as well as local capabilities of study sites, is key to enhance the efficiency of late-stage clinical development pf new antibiotics.
If you are interested in Ecraid’s warm-base network approach, read this excellent white paper on site selection procedures.
Figure: A map of the countries that were included in the COMBACTE-CARE studies through the CLIN-Net network.