Yes, according to this study, which was the talk of the summer.
In short: In mice experimental lung infection with SARS-CoV-2 and influenza reactivated dormant breast cancer cells, rapidly leading to metastatic disease accompanied by an inflammatory response resembling acute COVID-19 infection in humans.
And when translated from mice to men: “…analyses of cancer survivors from two cohorts reveal that SARS-CoV-2 infection substantially increases the risk of cancer related mortality and lung metastasis compared with uninfected cancer survivors.”
“… our studies reveal how respiratory virus infections can increase cancer recurrence risk and underscore the need for public health and clinical strategies to mitigate the increased risk of metastatic progression associated with SARS-CoV-2 and other respiratory virus infections.”
Although widely regarded as a scientific breakthrough and despite the firm conclusions, some emphasised that cancer survivors should not be alarmed yet.
From Mice to Men
What I missed (probably not seen all that was said) was a critical appraisal of the strength of the evidence translating the experimental findings in mice to real-world evidence in men.
The cohorts contained subjects that had survived cancer and were alive in January 2020:
• Cohort 1 (UK Biobank): 4,837 persons with any cancer diagnosis before 1 January 2015
• Cohort 2 (Flatiron Health): 36,845 female patients with breast cancer
These databases were linked to publicly available results from SARS-CoV-2 testing and death certificates up to December 2020, before vaccinations and self-testing began. Therefore, only the effects of COVID-19 during the first wave and the beginning of the second wave were analysed. During this first year of the pandemic, there was shortage of testing capacity, and persons needing healthcare for non-COVID-19 reasons had a higher likelihood of being tested, introducing detection bias.
The outcomes (all-cause mortality and cancer related mortality), based on the International Classification of Diseases (ICD) system, were analysed until December 2020, and in stepwise longer follow-up periods till December 2022.
Study validity
Misclassification may impact study validity. Classification of COVID-19 infection (YES or NO) was based on test results. In cohort 1 test-positive individuals (infected) were matched to test-negative individuals (not infected), partly controlling detection bias. Yet, a test result only reflects the presence or absence of infection at the time of testing. Test-negatives might have had COVID-19 at another time in 2020, and those having metastatic disease might have had a higher likelihood of being tested.
The first scenario could lead to overestimation, and the second to underestimation, of the calculated risks. Serologic testing of SARS-CoV-2-specific antibodies at the time of metastatic disease detection or in December 2020 would have prevented misclassification, but that data was not available.
Up to December 2020 there were 116 deaths (all-cause, of which 53 were cancer-related) yielding odds ratios of 14.03 (9.45-20.84) for all-cause and 8.24 (3.43 – 19.77) for cancer-related mortality among test-positives. In this analysis, infection, metastasis and death all must have occurred between March and December 2020.
Uncertainties in observational data
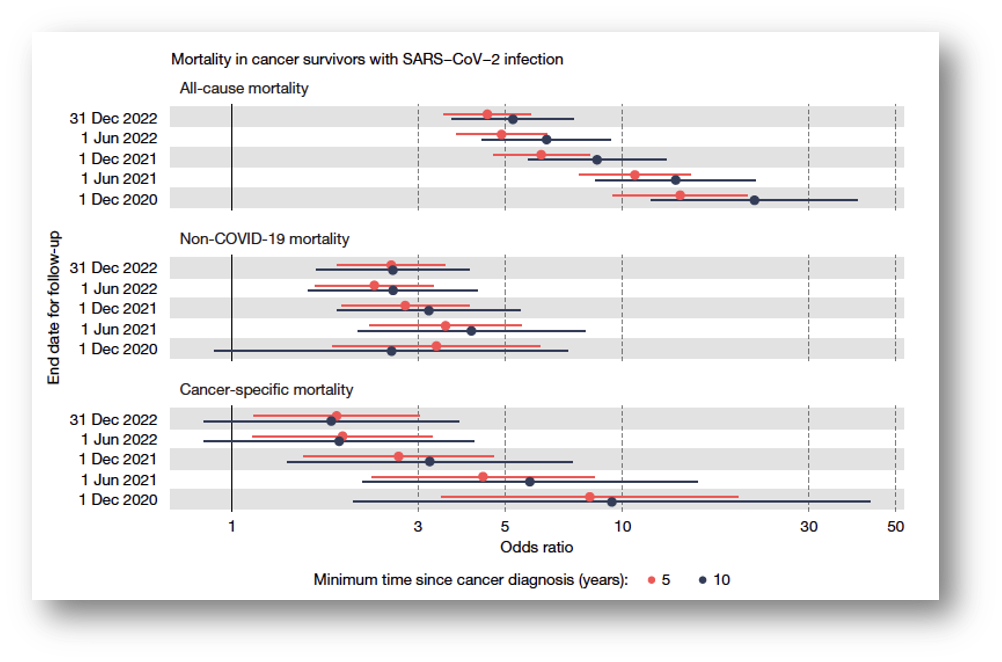
It is unknown how many of the 4,837 cancer survivors had breast cancer, how many had metastatic cancer, and how many died from metastasized cancer. Notably, odds ratios for both all-cause and cancer-related deaths declined when follow-up increased (see figure below), which is difficult to explain. Possible scenarios include:
• The awakened cells cause rapid mortality only
• Test-negative individuals became infected after December 2020 and subsequently died
• Vaccination (started in December 2020) prevented the onset of metastasis and death
• Vaccination produced the same effects of infection, exposing uninfected individuals to the same risk, thereby eliminating any risk difference.
Who can tell?
Cohort 2 comprised women with breast cancer but lacked information on negative test results. Therefore, all women with positive test results (n=589, 1.2%) were categorised as infected, and all without test results as non-infected.
Serology studies revealed that at the end of 2020, around 10% of the population in North America had been infected, suggesting that many women in this cohort were likely misclassified. This misclassification may have led to an underestimation of the hazard ratio of 1.44 (95% CI: 1.01, 2.05; P = 0.043), as stated by the authors. But then again, detection bias may have contributed to an overestimation of the risk.
Conclusion
It is impossible to fully adjust for these limitations, and these analyses are as good as it gets, with the available data. Such interesting findings, based on suboptimal data, are sometimes summarised as “if the trend is your friend.”
Careful interpretation is required as more studies are needed to confirm the results!