Randomised controlled trials (RCT) provide the highest level of evidence. But what to do if a disease changes? Only a new RCT can provide sufficient evidence to change the associated RCT-based practice.
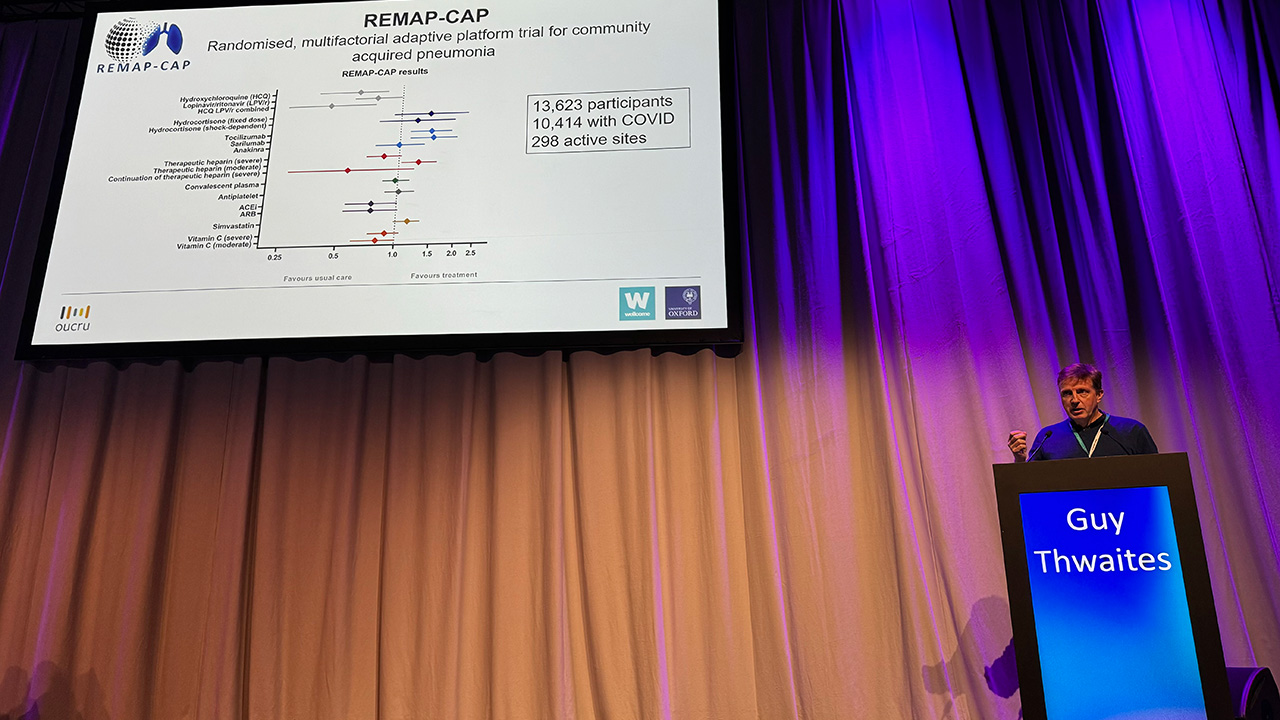
At ESCMID Global 2024, Guy Thwaites, one of Ecraid’s Scientific Advisory Board members, discussed “which randomised controlled trials do we need?” After recounting the history of RCTs and the “Oxford-bred” large trials in cardiovascular medicine, he used RECOVERY, REMAP-CAP and SNAP as examples of trials providing definitive answers for patients. He ended his talk with seven recommendations on how to revive the clinical trial ecosystem:
- Build stable and sustainable trial platforms/networks;
- Conduct large, pragmatic, academic trials addressing age-old questions;
- Invest in and strengthen regulatory agencies and their practices;
- Further develop academic-industry partnerships;
- Remove unwanted trial competition; coordinate and collaborate;
- Maximize trial efficiency without reducing quality (innovative, pragmatic trial designs)
- Stop using CRO’s: control costs and bureaucracy
Really nothing to add.
During the discussion afterwards, the question was raised whether Target Trial Emulation (TTE) could replace randomisation. For clarity, TTE is defined as “an observational research method that incorporates design features from idealized randomized trials (i.e., target trials) to improve the quality and interpretability of observational research.” My own interpretation has – sometimes – been, “selling a retrospective study with adjustments for covariates as an RCT”.
Guy Thwaites responded with “I remain unconvinced that there is any trial methodology that can replace randomisation.” The same conclusion as in a nice commentary in the European Journal of Epidemiology on a study comparing TTE in observational data to an RCT evaluating efficacy of beta-blocker use in patients with preserved left ventricular ejection fraction after a recent myocardial infarction.
So, if TTE cannot replace RCT, then only a new RCT can change practice that is based on “definitive” results from a prior RCT. But what if the disease has changed? The one example on my mind is immunomodulation for acute COVID-19. The RECOVERY and REMAP-CAP studies provided high-quality “definitive” evidence that corticosteroids, tocilizumab, and baricitinib improved outcomes in patients with acute severe COVID-19 in 2020 and early 2021. That was before vaccination and widespread natural boosts by omicron infections.
Since then, acute COVID-19 has changed dramatically: from “acute, rapidly progressive hypoxaemia leading to death, even in patients with minor comorbidity” to “a protracted deterioration of patients with multiple comorbidities and without the characteristic pulmonary findings”. Yet, these patients are still treated with immunomodulatory drugs, that may also have negative effects. How confident can we be that the treatment effects have remained unchanged?
Observational data cannot provide an answer. Instead, RECOVERY and REMAP-CAP could consider reopening their immunomodulation domains to investigate whether withholding of immunomodulation is non-inferior (or even superior). Just like clinicians once did with life-long co-trimoxazol for pneumocystis prophylaxis in HIV patients after the introduction of highly active antiretroviral therapies.
Which trial do you think we need to change RCT-based best practice?